Cardiac muscle
| cardiac muscle | |
|---|---|
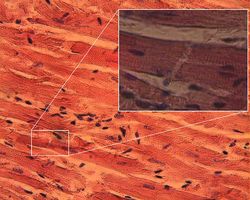 |
Cardiac muscle is a type of involuntary striated muscle found in the walls and histologic foundation of the heart, specifically the myocardium. Cardiac muscle is one of three major types of muscle, the others being skeletal and smooth muscle. The cells that comprise cardiac muscle are called cardiomyocytes and are sometimes seen as an intermediate between other types of muscle in terms of appearance, structure, metabolism, excitation-coupling and mechanism of contraction. Cardiac muscle shares similarities with skeletal muscle with regard to its striated appearance and contraction, with both differing significantly from smooth muscle cells. In addition, cardiac muscle cells, like skeletal muscle cells, are multinuclear whereas smooth muscle cells are mononuclear.[1]
Coordinated contraction of cardiac muscle cells in the heart propel blood forward from the atria and ventricles to the blood vessels of the circulatory system. Cardiac muscle cells, like all tissues in the body, rely on an ample blood supply to deliver oxygen and nutrients and to remove waste products such as carbon dioxide. The coronary arteries fulfill this function.

Contents |
Metabolism
Cardiac muscle is adapted to be highly resistant to Fatigue: it has a large number of mitochondria, enabling continuous aerobic respiration via oxidative phosphorylation, numerous myoglobins (oxygen-storing pigment) and a good blood supply, which provides nutrients and oxygen. The heart is so tuned to aerobic metabolism that it is unable to pump sufficiently in ischaemic conditions. At basal metabolic rates, about 1% of energy is derived from anaerobic metabolism. This can increase to 10% under moderately hypoxic conditions, but, under more severe hypoxic conditions, not enough energy can be liberated by lactate production to sustain ventricular contractions.[2]
Under basal aerobic conditions, 60% of energy comes from fat (free fatty acids and triglycerides), 35% from carbohydrates, and 5% from amino acids and ketone bodies. However, these proportions vary widely according to nutritional state. For example, during starvation, lactate can be recycled by the heart. This is very energy efficient, because one NAD+ is reduced to NADH and H+ (equal to 2.5 or 3 ATP) when lactate is oxidized to pyruvate, which can then be burned aerobically in the TCA cycle, liberating much more energy (ca 14 ATP per cycle).
In the condition of diabetes, more fat and less carbohydrate is used due to the reduced induction of GLUT4 glucose transporters to the cell surfaces. However, contraction itself plays a part in bringing GLUT4 transporters to the surface.[3] This is true of skeletal muscle as well, but relevant in particular to cardiac muscle due to its continuous contractions.
Appearance
Striation
Cardiac muscle exhibits cross striations formed by alternating segments of thick and thin protein filaments. Like skeletal muscle, the primary structural proteins of cardiac muscle are actin and myosin. The actin filaments are thin causing the lighter appearance of the I bands in striated muscle, while the myosin filament is thicker lending a darker appearance to the alternating A bands as observed with electron microscopy. However, in contrast to skeletal muscle, cardiac muscle cells may be branched instead of linear and longitudinal.
T-Tubules
Another histological difference between cardiac muscle and skeletal muscle is that the T-tubules in cardiac muscle are larger, broader and run along the Z-Discs. There are fewer T-tubules in comparison with skeletal muscle. Additionally, cardiac muscle forms diads instead of the triads formed between the T-tubules and the sarcoplasmic reticulum in skeletal muscle. T-tubules play critical role in excitation-contraction coupling (ECC). Recently, the action potentials of T-tubules were recorded optically by Guixue Bu et al.[4]
Intercalated discs
Intercalated discs (IDs) are complex adhering structures which connect single cardiac myocytes to an electrochemical syncytium (in contrast to the skeletal muscle, which becomes a multicellular syncytium during mammalian embryonic development) and are mainly responsible for force transmission during muscle contraction. Intercalated discs also support the rapid spread of action potentials and the synchronized contraction of the myocardium. IDs are described to consist of three different types of cell-cell junctions: the actin filament anchoring adherens junctions (fascia adherens), the intermediate filament anchoring desmosomes (macula adherens) and gap junctions. Gap junctions are responsible for electrochemical and metabolic coupling. They allow action potentials to spread between cardiac cells by permitting the passage of ions between cells, producing depolarization of the heart muscle. However, novel molecular biological and comprehensive studies unequivocally showed that IDs consist for the most part of mixed type adhering junctions named area composita (pl. areae compositae) representing an amalgamation of typical desmosomal and fascia adhaerens proteins (in contrast to various epithelia). The authors discuss the high importance of these findings for the understanding of inherited cardiomyopathies (such as Arrhythmogenic Right Ventricular Cardiomyopathy, ARVC).
Under light microscopy, intercalated discs appear as thin, typically dark-staining lines dividing adjacent cardiac muscle cells. The intercalated discs run perpendicular to the direction of muscle fibers. Under electron microscopy, an intercalated disc's path appears more complex. At low magnification, this may appear as a convoluted electron dense structure overlying the location of the obscured Z-line. At high magnification, the intercalated disc's path appears even more convoluted, with both longitudinal and transverse areas appearing in longitudinal section.[5]
Role of calcium in contraction
In contrast to skeletal muscle, cardiac muscle requires extracellular calcium ions for contraction to occur. Like skeletal muscle, the initiation and upshoot of the action potential in ventricular muscle cells is derived from the entry of sodium ions across the sarcolemma in a regenerative process. However, an inward flux of extracellular calcium ions through L-type calcium channels sustains the depolarization of cardiac muscle cells for a longer duration. The reason for the calcium dependence is due to the mechanism of calcium-induced calcium release (CICR) from the sarcoplasmic reticulum that must occur under normal excitation-contraction (EC) coupling to cause contraction. Once the intracellular concentration of calcium increases, calcium ions bind to the protein troponin, which initiates contraction by allowing the contractile proteins, myosin and actin to associate through cross-bridge formation. Cardiac muscle is intermediate between smooth muscle, which has an unorganized sarcoplasmic reticulum and derives its calcium from both the extracellular fluid and intracellular stores, and skeletal muscle, which is only activated by calcium stored in the sarcoplasmic reticulum.
Regeneration of heart muscle cells
Until recently, it was commonly believed that cardiac muscle cells could not be regenerated. However, a study reported in the April 3, 2009 issue of Science contradicts that belief.[6] Olaf Bergmann and his colleagues at the Karolinska Institute in Stockholm tested samples of heart muscle from people born before 1955 when nuclear bomb testing caused elevated levels of radioactive carbon 14 in the Earth's atmosphere. They found that samples from people born before 1955 did have elevated carbon 14 in their heart muscle cell DNA, indicating that the cells had divided after the person's birth. By using DNA samples from many hearts, the researchers estimated that a 20-year-old renews about 1% of heart muscle cells per year and about 45 percent of the heart muscle cells of a 50-year-old were generated after he or she was born.
See also
- Heart
- Circulatory system
- Cardiac action potential
- Calcium sparks
- Troponin
References
- ↑ University of Guelph Developmental Biology ONLINE! web site (retrieved 2010-05-04) http://www.uoguelph.ca/zoology/devobio/210labs/muscle1.html
- ↑ Ganong, Review of Medical Physiology, 22nd Edition.Specialized form of muscle that is peculiar to the vertebrate heart.p81
- ↑ S Lund, GD Holman, O Schmitz, and O Pedersen. Contraction Stimulates Translocation of Glucose Transporter GLUT4 in Skeletal Muscle Through a Mechanism Distinct from that of Insulin. PNAS 92: 5817-5821.
- ↑ Guixue Bu, et al. Uniform action potential repolarization within the sarcolemma of in situ ventricular cardiomyocytes. Biophysical Journal, Vol.96, No.6, March 2009, pp.2532-2546.
- ↑ Histology at BU 22501loa
- ↑ Evidence for cardiomyocyte renewal in humans. PMID 19342590
- Lodish, H., Berk, A., Zipursky, L. S., Matsudaira, P., Baltimore, D., Darnell, J. 2000. Molecular Cell Biology. ISBN 0-7167-3136-3 1
- Franke, W. W., Borrmann, C. M. ,Grund, C., Pieperhoff, S., 2006. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol 85, 69-82.
- Borrmann, C. M., Grund, C., Kuhn, C., Hofmann, I., Pieperhoff, S., Franke, W. W., 2006. The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. Eur J Cell Biol 85, 469-85.
- Pieperhoff, S., Franke, W. W., 2007. The area composita of adhering junctions connecting heart muscle cells of vertebrates - IV: Coalescence and amalgamation of desmosomal and adhaerens junction components - Late processes in mammalian heart development. Eur J Cell Biol 86, 377-91.
- Pieperhoff, S., Franke, W. W., 2008. The area composita of adhering junctions connecting heart muscle cells of vertebrates -VI. Polar and lateral junctions of non-mammalian species. Eur J Cell Biol. 87, 413-30.
- Shimada, T., Kawazato, H., Yasuda, A., Ono, N., Sueda, K., 2004. Cytoarchitecture and intercalated disks of the working myocardium and the conduction system in the mammalian heart. Anat Rec A Discov Mol Cell Evol Biol. 280, 940-51.
- Waschke, J., 2008. The desmosome and pemphigus. Histochem Cell Biol 130, 21-54.
External links
- Indiana State University, Muscle action
- Physiology at MCG 2/2ch7/2ch7line
|
||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||